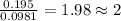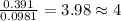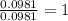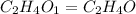Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
The foul odor of rancid butter is due largely to butyric acid, a compound containing carbon, hydroge...
Questions


Mathematics, 14.12.2020 07:50

Geography, 14.12.2020 07:50


Mathematics, 14.12.2020 07:50

Chemistry, 14.12.2020 07:50

Mathematics, 14.12.2020 07:50




English, 14.12.2020 07:50





Mathematics, 14.12.2020 07:50

History, 14.12.2020 07:50

Social Studies, 14.12.2020 07:50

Advanced Placement (AP), 14.12.2020 07:50

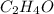
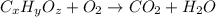
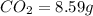
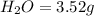
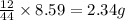 of carbon will be contained.
of carbon will be contained.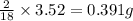 of hydrogen will be contained.
of hydrogen will be contained.![(4.30g)-[(2.34g)+(0.391g)]=1.57g](/tpl/images/0308/8464/24f5b.png)
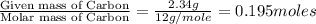
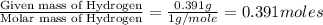
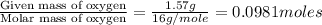
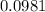 moles.
moles.