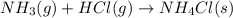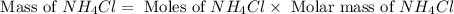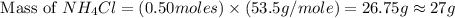For each of the following balanced chemical equations, calculate how many moles and how many grams of each product would be produced by the complete conversion of 0.50 mole of the reactant indicated in boldface. state clearly the mole ratio used for each conversion. a. nh3(g) 1 hcl(g) s nh4cl(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1

Chemistry, 23.06.2019 17:00
During which of the following phases of the moon do we see the left half of the moon as lit? full moon first quarter moon gibbous moon third quarter moon any is greatly : )
Answers: 1

Chemistry, 23.06.2019 17:30
If 2.40 moles of gas are held at a temperature of 97.0 °c in a container with a volume of 45.0 l, what is the pressure of the gas?
Answers: 1
You know the right answer?
For each of the following balanced chemical equations, calculate how many moles and how many grams o...
Questions

Computers and Technology, 24.02.2021 20:30

History, 24.02.2021 20:30

English, 24.02.2021 20:30


Mathematics, 24.02.2021 20:30

Mathematics, 24.02.2021 20:30



Mathematics, 24.02.2021 20:30

Mathematics, 24.02.2021 20:30


Geography, 24.02.2021 20:30


English, 24.02.2021 20:30

Mathematics, 24.02.2021 20:30


Mathematics, 24.02.2021 20:30

History, 24.02.2021 20:30

Mathematics, 24.02.2021 20:30

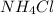 and mass of
and mass of 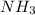 . So,
. So,