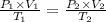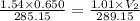Abubble of helium gas has a volume of 0.650 cm3 near the bottom of a large aquarium where the pressure is 1.54 atm and the temperature is 12°c. determine the bubble’s volume upon rising near the top where the pressure is 1.01 atm and 16°c. assume that the number of moles of helium remains constant and that the helium is an ideal gas. (13 pts)
important equations and constants 1 atm = 760 torr = 760 mmhg = 101,325 pa
1ml = 1cm3
pv = nrt
p1v1 = p2v2
v1/t1 = v2/t2
v1/n1 = v2/n2
ptotal = p1 + p2 + p3 + ….
(p1v1)/(n1t1) = (p2v2)/(n2t2)
r = 0.08206 l atm/mol k

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Abubble of helium gas has a volume of 0.650 cm3 near the bottom of a large aquarium where the pressu...
Questions

English, 04.02.2021 03:30

Mathematics, 04.02.2021 03:30




Mathematics, 04.02.2021 03:30


Social Studies, 04.02.2021 03:30

Advanced Placement (AP), 04.02.2021 03:30

Arts, 04.02.2021 03:30

Mathematics, 04.02.2021 03:30


Mathematics, 04.02.2021 03:30

Mathematics, 04.02.2021 03:30

Social Studies, 04.02.2021 03:30


Geography, 04.02.2021 03:30

Social Studies, 04.02.2021 03:30

Mathematics, 04.02.2021 03:30

Health, 04.02.2021 03:30