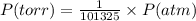Chemistry, 10.10.2019 05:20 hillarytrinh
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, are injected into an evacuated 536 cm3 flask.
a. find the number of moles of oxygen present prior to mixing the gases, assuming the temperature remains constant, and that oxygen is an ideal gas. (5pts)
b. find the number of moles of nitrogen present prior to mixing the gases, assuming the temperature remains constant and that nitrogen is an ideal gas. (5pts)
c. find the total pressure of oxygen and nitrogen in the flask (after the two gases are mixed together.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2


You know the right answer?
495 cm3 of oxygen gas at 25 oc and 114,700 pa, and 877 cm3 of nitrogen gas 25.0 °c and 114,700 pa, a...
Questions

Chemistry, 25.01.2020 12:31

Mathematics, 25.01.2020 12:31




Chemistry, 25.01.2020 12:31



History, 25.01.2020 12:31



History, 25.01.2020 12:31

Mathematics, 25.01.2020 12:31


Biology, 25.01.2020 12:31

English, 25.01.2020 12:31


Mathematics, 25.01.2020 12:31

Mathematics, 25.01.2020 12:31