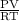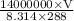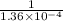Chemistry, 10.10.2019 03:20 memoryofdale
Apressure gauge at the top of a vertical oil well registers 140 bars (=14 mpa). the oil well is 6000 m deep and filled with natural gas down to a depth of 4700 m and filled with oil (density=700 kg/m') the rest of the way to the bottom of the well at 15°c. the compressibility factor z=0.80 for natural gas and its molecular weight is 18.9. determine the pressure at (a) the natural gas-oil interface and at (b) the bottom of the well at 15°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1


Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Apressure gauge at the top of a vertical oil well registers 140 bars (=14 mpa). the oil well is 6000...
Questions



Engineering, 04.07.2019 19:10








Geography, 04.07.2019 19:10



Mathematics, 04.07.2019 19:10


Business, 04.07.2019 19:10

Mathematics, 04.07.2019 19:10



Engineering, 04.07.2019 19:10