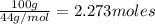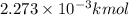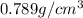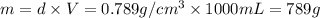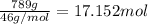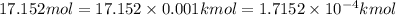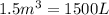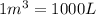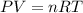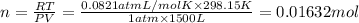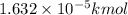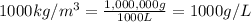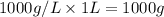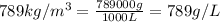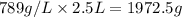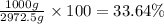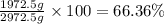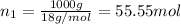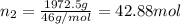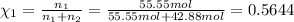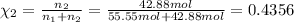How many moles, kmols in: 100 g of co2, 1 litre of ethyl alcohol of density 0.789 g/cm3 and a) 1.5m3 of o2 at 25°c and 1 atm. b) a mixture of water and ethyl alcohol is made up of 1 litre of water and 2.5 litre of alcohol. calculate the mass fraction and mol fraction for water and alcohol density of water 1000 kg/m density of alcohol 789 kg/m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 1
You know the right answer?
How many moles, kmols in: 100 g of co2, 1 litre of ethyl alcohol of density 0.789 g/cm3 and a) 1.5m...
Questions



Mathematics, 16.12.2020 16:50

History, 16.12.2020 16:50










Mathematics, 16.12.2020 16:50

English, 16.12.2020 16:50

English, 16.12.2020 16:50