
Chemistry, 08.10.2019 18:20 memester74
The equilibrium constant k for a certain reversible reaction is 1.32 × 10−4. which of the following is true for the reaction? the reverse reaction is more favorable than the forward reaction. the forward reaction takes place at a much faster rate than the reverse reaction. the equilibrium reaction mixture will have a very low concentration of reactants. the equilibrium reaction mixture will have a very high concentration of products.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

You know the right answer?
The equilibrium constant k for a certain reversible reaction is 1.32 × 10−4. which of the following...
Questions


Geography, 19.11.2020 18:40


Mathematics, 19.11.2020 18:40




Mathematics, 19.11.2020 18:40

Chemistry, 19.11.2020 18:40


Health, 19.11.2020 18:40


Physics, 19.11.2020 18:40




Mathematics, 19.11.2020 18:40



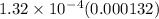 . A high value of equilibrium constant indicates that the forward reaction is more favourable.
. A high value of equilibrium constant indicates that the forward reaction is more favourable. 

