
Chemistry, 08.10.2019 03:30 duncanje5783
Be sure to answer all parts. determine the overall orders of the reactions to which the following rate laws apply: (a) rate = k[no2]2 (b) rate = k zero order first order 1.5 order second order 2.5 order third order zero order first order 1.5 order second order 2.5 order third order (c) rate = k[h2][br2]1/2 (d) rate = k[no2]2[o2] zero order first order 1.5 order second order 2.5 order third order zero order first order 1.5 order second order 2.5 order third order

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

You know the right answer?
Be sure to answer all parts. determine the overall orders of the reactions to which the following ra...
Questions

History, 08.04.2020 23:17

English, 08.04.2020 23:17

History, 08.04.2020 23:17

Biology, 08.04.2020 23:17

Mathematics, 08.04.2020 23:17


English, 08.04.2020 23:17





Mathematics, 08.04.2020 23:17

Mathematics, 08.04.2020 23:17




Mathematics, 08.04.2020 23:17


English, 08.04.2020 23:17

Mathematics, 08.04.2020 23:17

![r=k[NO_2]^2](/tpl/images/0299/0794/8772b.png)
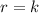
![r=k[H_2]*[Br_2]^{1/2}](/tpl/images/0299/0794/34ab2.png)
![r=k[NO_2]^2[O_2]](/tpl/images/0299/0794/ccd53.png)


