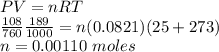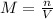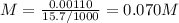The volume of a sample of pure hcl gas was 189 ml at 25°c and 108 mmhg. it was completely dissolved in about 60 ml of water and titrated with an naoh solution; 15.7 ml of the naoh solution were required to neutralize the hcl. calculate the molarity of the naoh solution.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
The volume of a sample of pure hcl gas was 189 ml at 25°c and 108 mmhg. it was completely dissolved...
Questions




English, 04.06.2020 19:12




Physics, 04.06.2020 19:12

Mathematics, 04.06.2020 19:12

Mathematics, 04.06.2020 19:12

Mathematics, 04.06.2020 19:12

Mathematics, 04.06.2020 19:12


Mathematics, 04.06.2020 19:12

English, 04.06.2020 19:12




Mathematics, 04.06.2020 19:12

Mathematics, 04.06.2020 19:12