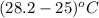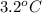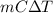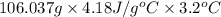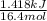Acommon laboratory reaction is the neutralization of an acid with a base. when 32.8 ml of 0.500 m hcl at 25.0°c is added to 66.3 ml of 0.500 m naoh at 25.0°c in a coffee cup calorimeter (with a negligible heat capacity), the temperature of the mixture rises to 28.2°c. what is the heat of reaction per mole of nacl (in kj/mol)? assume the mixture has a specific heat capacity of 4.18 j/(g·k) and that the densities of the reactant solutions are both 1.07 g/ml.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Acommon laboratory reaction is the neutralization of an acid with a base. when 32.8 ml of 0.500 m hc...
Questions

Social Studies, 11.06.2020 19:57

English, 11.06.2020 19:57

Mathematics, 11.06.2020 19:57


History, 11.06.2020 19:57

Biology, 11.06.2020 19:57

Biology, 11.06.2020 19:57

English, 11.06.2020 19:57

Social Studies, 11.06.2020 19:57

Physics, 11.06.2020 19:57


Spanish, 11.06.2020 19:57

Mathematics, 11.06.2020 19:57

Biology, 11.06.2020 19:57


Mathematics, 11.06.2020 19:57

Geography, 11.06.2020 19:57

Mathematics, 11.06.2020 19:57


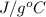

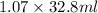
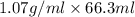
 =
=