
A5.00-ml sample of blood was treated with trichloroacetic acid to precipitate proteins. after centrifugation, the resulting solution was brought to a ph of 3 and was extracted with two 5-ml portions of methyl isobutyl ketone containing the organic lead complexing agent apcd. the extract was aspirated directly into an air-acetylene flame yielding an absorbance of 0.454 at 283.3 nm. five-milliliter aliquots of standard solutions containing 0.240 and 0.475 ppm pb were treated in the same way and yielded absorbances of 0.412 and 0.642. calculate the concentration pb (ppm) in the sample assuming that beer’s law is followed.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
A5.00-ml sample of blood was treated with trichloroacetic acid to precipitate proteins. after centri...
Questions


History, 03.12.2020 06:50

History, 03.12.2020 06:50

French, 03.12.2020 06:50


Mathematics, 03.12.2020 06:50

Mathematics, 03.12.2020 06:50

Geography, 03.12.2020 06:50

Mathematics, 03.12.2020 07:00


Mathematics, 03.12.2020 07:00

Arts, 03.12.2020 07:00

Mathematics, 03.12.2020 07:00



English, 03.12.2020 07:00

Mathematics, 03.12.2020 07:00

Mathematics, 03.12.2020 07:00


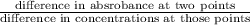
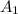 = 0.412 (first point)
= 0.412 (first point)
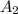 = 0.642 (second point)
= 0.642 (second point)
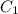 = 0.240 ppm (first point)
= 0.240 ppm (first point)
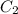 = 0.475 ppm (second point)
= 0.475 ppm (second point)
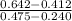
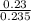
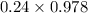 + c
+ c
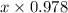 + 0.178
+ 0.178

