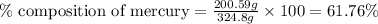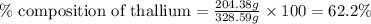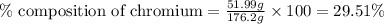Chemistry, 07.10.2019 20:00 hala201490
Be sure to answer all parts. dimercaprol (hsch2chshch2oh) was developed during world war i as an antidote to arsenic-based poison gas and is used today to treat heavy-metal poisoning. it binds the toxic element and carries it out of the body. (a) if each molecule binds one arsenic (as) atom, how many atoms of as could be removed by 696 mg of dimercaprol? × 10 atoms as (enter your answer in scientific notation.) (b) if one molecule binds one metal atom, calculate the mass % of each of the following metals in a metal-dimercaprol combination: mercury, thallium, chromium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
Be sure to answer all parts. dimercaprol (hsch2chshch2oh) was developed during world war i as an ant...
Questions

History, 25.12.2019 00:31

History, 25.12.2019 00:31


Biology, 25.12.2019 00:31

Social Studies, 25.12.2019 00:31

Biology, 25.12.2019 00:31

History, 25.12.2019 00:31


Mathematics, 25.12.2019 00:31







Mathematics, 25.12.2019 00:31

Social Studies, 25.12.2019 00:31


Mathematics, 25.12.2019 00:31

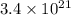
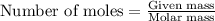
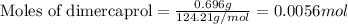
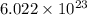 number of molecules.
number of molecules.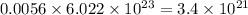 number of molecules.
number of molecules. number of arsenic atoms
number of arsenic atoms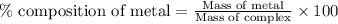 ......(1)
......(1)