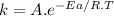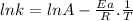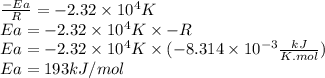Chemistry, 05.10.2019 04:20 Dreynolds1667
For the gas phase isomerization of cis-cyanostyrene, cis-c6h5ch=chc --> ntrans-c6h5ch=chcn the rate constant has been determined at several temperatures. when ln k in s-1 is plotted against the reciprocal of the kelvin temperature, the resulting linear plot has a slope of -2.32×104 k and a y-intercept of 26.7. the activation energy for the gas phase isomerization of cis-cyanostyrene is kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
For the gas phase isomerization of cis-cyanostyrene, cis-c6h5ch=chc --> ntrans-c6h5ch=chcn the r...
Questions

World Languages, 30.12.2019 06:31


Mathematics, 30.12.2019 06:31

Geography, 30.12.2019 06:31




Business, 30.12.2019 06:31

Mathematics, 30.12.2019 06:31



Health, 30.12.2019 06:31

Mathematics, 30.12.2019 06:31


Social Studies, 30.12.2019 06:31

Mathematics, 30.12.2019 06:31



Biology, 30.12.2019 06:31