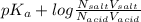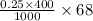Chemistry, 05.10.2019 04:20 Sumitco9578
Calculate how to prepare 750 ml of 0.25 m sodium formate buffer at ph 4. use your textbook to determine the molecular weight and pka of the acid and base. calculate the grams of sodium formate and number of milliliters of formic acid required. then using this stock solution, calculate and describe how you would prepare 100 ml of a 10 mm formate buffer, ph 3.5. by the way, what is the molarity of formic acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
Calculate how to prepare 750 ml of 0.25 m sodium formate buffer at ph 4. use your textbook to determ...
Questions


Chemistry, 02.03.2020 21:25



Biology, 02.03.2020 21:25



Mathematics, 02.03.2020 21:25


Mathematics, 02.03.2020 21:25








Computers and Technology, 02.03.2020 21:25


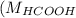 = 46 g/mol
= 46 g/mol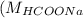 = 68 g/mol
= 68 g/mol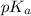 of HCOOH = 3.75
of HCOOH = 3.75![[HCOO^{-}]](/tpl/images/0288/1557/bee9e.png) = [HCOOH] + [HCOONa]
= [HCOOH] + [HCOONa]