
Chemistry, 05.10.2019 04:10 shels10tay
Be sure to answer all parts. write the balanced equations corresponding to the following rate expressions: a) rate = − 1 3 δ[ch4] δt = − 1 2 δ[h2o] δt = − δ[co2] δt = 1 4 δ[ch3oh] δt (click in the answer box to activate the palette. do not include states of matter.) b) rate = − 1 2 δ[n2o5] δt = 1 2 δ[n2] δt = 1 5 δ[o2] δt (click in the answer box to activate the palette. do not include states of matter.) c) rate = − 1 2 δ[h2] δt = − 1 2 δ[co2] δt = − δ[o2] δt = 1 2 δ[h2co3] δt (click in the answer box to activate the palette. do not include states of matter.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
Be sure to answer all parts. write the balanced equations corresponding to the following rate expres...
Questions

English, 06.06.2021 14:00

Biology, 06.06.2021 14:00


Biology, 06.06.2021 14:00

Biology, 06.06.2021 14:00

Mathematics, 06.06.2021 14:00


Mathematics, 06.06.2021 14:00



Mathematics, 06.06.2021 14:00

Biology, 06.06.2021 14:00


English, 06.06.2021 14:00

Mathematics, 06.06.2021 14:00



Chemistry, 06.06.2021 14:00

Mathematics, 06.06.2021 14:00

Mathematics, 06.06.2021 14:00

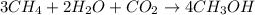
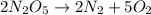
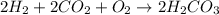
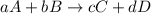
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0288/1459/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0288/1459/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0288/1459/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0288/1459/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0288/1459/d4b94.png)
![Rate=-\frac{1}{3}\frac{d[CH_4]}{dt}=-\frac{1}{2}\frac{d[H_2O]}{dt}=-\frac{d[CO_2]}{dt}=+\frac{1}{4}\frac{d[CH_3OH]}{dt}](/tpl/images/0288/1459/a1c90.png)
![Rate=-\frac{1}{2}\frac{d[N_2O_5]}{dt}=+\frac{1}{2}\frac{d[N_2]}{dt}=+\frac{1}{5}\frac{d[O_2]}{dt}](/tpl/images/0288/1459/697db.png)
![Rate=-\frac{1}{2}\frac{d[H_2]}{dt}=-\frac{1}{2}\frac{d[CO_2]}{dt}=-\frac{d[O_2]}{dt}=+\frac{1}{2}\frac{d[H_2CO_3]}{dt}](/tpl/images/0288/1459/13070.png)


