
Chemistry, 06.10.2019 10:00 hannahmorgret7811
Consider the following reaction between mercury(ii) chloride and oxalate ion.
2 hgcl2(aq) + c2o42-(aq) 2 cl -(aq) + 2 co2(g) + hg2cl2(s)
the initial rate of this reaction was determined for several concentrations of hgcl2 and c2o42-, and the following rate data were obtained for the rate of disappearance of c2o42-.
experiment [hgcl2] (m) [c2o42-] (m) rate (m/s)
1 0.164 0.15 3.2x10^-5
2 0.164 0.45 2.9x10^-4
3 0.082 0.45 1.4x10^-4
4 0.246 0.15 4.8x10^-5
what is the rate law for this reaction?
(a) -k[hgcl2][c2o4-2]2-
(b) -k[hgcl2]2[c2o4-2]
(c) -k[hgcl2]2[c2o4-2]1/2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Consider the following reaction between mercury(ii) chloride and oxalate ion.
2 hgcl2(aq...
2 hgcl2(aq...
Questions






Mathematics, 10.05.2021 19:30



History, 10.05.2021 19:30









Biology, 10.05.2021 19:30


![\text{Rate}=k[HgCl_2][C_2O_4^{2-}]^2](/tpl/images/0293/1267/42a62.png)
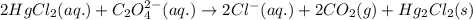
![\text{Rate}=k[HgCl_2]^a[C_2O_4^{2-}]^b](/tpl/images/0293/1267/af610.png)
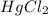
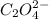
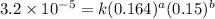 ....(1)
....(1)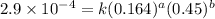 ....(2)
....(2)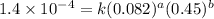 ....(3)
....(3)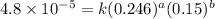 ....(4)
....(4) 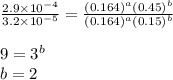
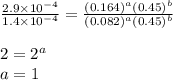
![\text{Rate}=k[HgCl_2]^1[C_2O_4^{2-}]^2](/tpl/images/0293/1267/956be.png)


