
Chemistry, 06.10.2019 10:00 cjrogers4401
You are asked to prepare 500 ml 0.300 m500 ml 0.300 m acetate buffer at ph 4.904.90 using only pure acetic acid ( mw=60.05 g/mol, mw=60.05 g/mol, pka=4.76), pka=4.76), 3.00 m naoh,3.00 m naoh, and water. answer the questions regarding the preparation of the buffer. 1. how many grams of acetic acid will be needed to prepare the 500 ml buffer? note that the given concentration of acetate refers to the concentration of all acetate species in solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3
You know the right answer?
You are asked to prepare 500 ml 0.300 m500 ml 0.300 m acetate buffer at ph 4.904.90 using only pure...
Questions


Mathematics, 10.12.2020 14:00

Computers and Technology, 10.12.2020 14:00


History, 10.12.2020 14:00




Engineering, 10.12.2020 14:00

Chemistry, 10.12.2020 14:00

Geography, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

Computers and Technology, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00




![\frac{[A^{-}]}{[HA]}](/tpl/images/0293/1269/5980f.png)
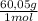 = 9,0 g of acetic acid
= 9,0 g of acetic acid

