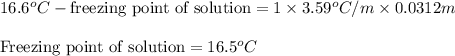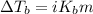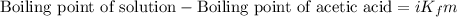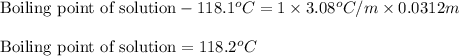Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 16:20
Which of the following subject areas contains questions that can be answered by science? alchemy ethics forensics politics
Answers: 3
You know the right answer?
Asolution was prepared by dissolving 0.800 g of sulfur s8, in 100.0 g of acetic acid, hc2h3o2. calcu...
Questions

Mathematics, 11.02.2022 22:30

Mathematics, 11.02.2022 22:30

Mathematics, 11.02.2022 22:30

Biology, 11.02.2022 22:30


Mathematics, 11.02.2022 22:30

Medicine, 11.02.2022 22:30

Mathematics, 11.02.2022 22:30


Mathematics, 11.02.2022 22:30

Physics, 11.02.2022 22:30



English, 11.02.2022 22:40

Mathematics, 11.02.2022 22:40


Mathematics, 11.02.2022 22:40



Health, 11.02.2022 22:40

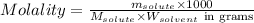
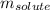 = Given mass of solute
= Given mass of solute 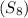 = 0.800 g
= 0.800 g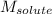 = Molar mass of solute
= Molar mass of solute  = 256.52 g/mol
= 256.52 g/mol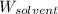 = Mass of solvent (acetic acid) = 100.0 g
= Mass of solvent (acetic acid) = 100.0 g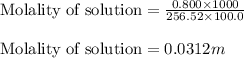
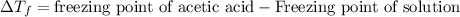
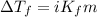
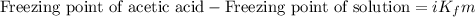
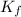 = molal freezing point depression constant = 3.59°C/m
= molal freezing point depression constant = 3.59°C/m