
Chemistry, 02.10.2019 22:00 hunterwilliams375
What is the vapor pressure at 23 oc of a solution of 1.20 g of naphthalene, c10h8, in 25.6 g of benzene, c6h6? . the vapor pressure of pure benzene at 23 oc is 86.0 mm hg; the vapor pressure of naphthalene can be neglected. calculate the vapor-pressure lowering of the solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
What is the vapor pressure at 23 oc of a solution of 1.20 g of naphthalene, c10h8, in 25.6 g of benz...
Questions

Mathematics, 07.06.2021 22:10

Mathematics, 07.06.2021 22:10

Chemistry, 07.06.2021 22:10

English, 07.06.2021 22:10

Social Studies, 07.06.2021 22:10


Business, 07.06.2021 22:10





Mathematics, 07.06.2021 22:10

English, 07.06.2021 22:10

Computers and Technology, 07.06.2021 22:10



Mathematics, 07.06.2021 22:10

World Languages, 07.06.2021 22:10


Mathematics, 07.06.2021 22:10

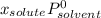 (1)
(1)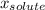 is molar fraction of solute
is molar fraction of solute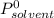 is the capor pressure of the pure solvent (86,0 mmHg)
is the capor pressure of the pure solvent (86,0 mmHg)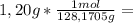 9,36x10⁻³ moles solute
9,36x10⁻³ moles solute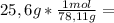 0,328 moles solvent
0,328 moles solvent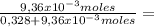 0,0278
0,0278

