
Chemistry, 02.10.2019 21:00 sahaitong1844
Specific heat material copper aluminum gold pyrex specific heat (j/g °c) 0.385 0.902 0.129 0.75 material water ice olive oil air specific heat (1/g °c) 4.18 2.01 2.38 1.00 if the same amount of heat was applied to a vessel containing 100.0 g of water and 100.0 g of olive oil, which would get hotter? why? if you had a 1.0 kg pot made of each metal, which pot would allow you to heat water to boiling more quickly? why? how much heat would it take to raise the temperature of 100.0 g of water from 20.0°c to 100.0 °c? (ignore the pot)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
Specific heat material copper aluminum gold pyrex specific heat (j/g °c) 0.385 0.902 0.129 0.75 mate...
Questions

Mathematics, 02.04.2020 23:29



History, 02.04.2020 23:29

Mathematics, 02.04.2020 23:29




Mathematics, 02.04.2020 23:29





Social Studies, 02.04.2020 23:29

Mathematics, 02.04.2020 23:29

Mathematics, 02.04.2020 23:29

Mathematics, 02.04.2020 23:30

Mathematics, 02.04.2020 23:30


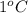 is known as specific heat.
is known as specific heat.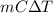
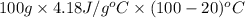
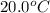 to
to 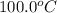 .
.


