
Chemistry, 01.10.2019 03:30 khill21208
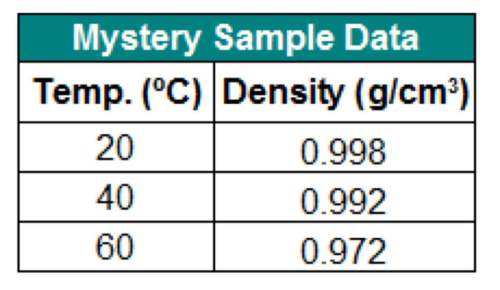
The table below shows the density of a sample of a mystery liquid you tested in the lab. can you infer the identity of the substance from these data?
a. yes, the substance must be water.
b. no, more data are needed.
c. no, the data must be wrong because density always decreases with an increase in temperature.
d. yes, but only if the data for 50ºc and 70ºc were also present.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1
You know the right answer?
The table below shows the density of a sample of a mystery liquid you tested in the lab. can you inf...
Questions


Mathematics, 20.08.2019 12:50







Biology, 20.08.2019 12:50



Mathematics, 20.08.2019 12:50

Mathematics, 20.08.2019 12:50


Biology, 20.08.2019 12:50

Health, 20.08.2019 12:50


Mathematics, 20.08.2019 12:50


Mathematics, 20.08.2019 12:50



