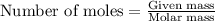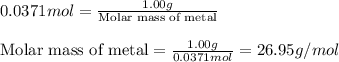Chemistry, 28.09.2019 02:10 sierranicole114
A1.00 g sample of a metal x (that is known to form x ions in solution) was added to 127.9 ml of 0.5000 m sulfuric acid. after all the metal had reacted, the remaining acid required 0.03340 l of 0.5000 m naoh solution for complete neutralization. calculate the molar mass of the metal and identify the element.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
A1.00 g sample of a metal x (that is known to form x ions in solution) was added to 127.9 ml of 0.50...
Questions

Mathematics, 10.02.2021 01:00



Chemistry, 10.02.2021 01:00


History, 10.02.2021 01:00

Mathematics, 10.02.2021 01:00

Mathematics, 10.02.2021 01:00

Mathematics, 10.02.2021 01:00

English, 10.02.2021 01:00

Health, 10.02.2021 01:00

Mathematics, 10.02.2021 01:00



Mathematics, 10.02.2021 01:00

Mathematics, 10.02.2021 01:00


Biology, 10.02.2021 01:00

Mathematics, 10.02.2021 01:00

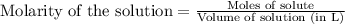 .....(1)
.....(1)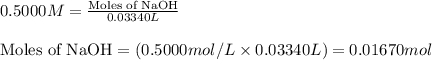

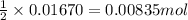 of sulfuric acid
of sulfuric acid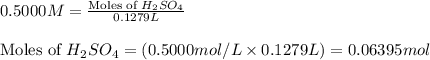
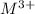 ion) and sulfuric acid follows:
ion) and sulfuric acid follows: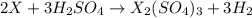
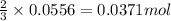 of metal
of metal