
Chemistry, 28.09.2019 02:10 Sylnatius6736
4. al2o3 (s) + 6hcl (aq) → 2alcl3 (aq) + 3h20(1) find the mass of alcl3 that is produced when 10.0 grams of al2o3 react with 10.0 g of hci moar mass alqo3 102 gm imol al2o3 = 0.098 moles molar mass of hcl = 36, 5gr/mol #of moles #6l =0,274 moles mole of al2 oz 6 mol of hc 01274 x2 = 0.0913 moles alc3=13,5 6 i mass alco3 = 12, 193gm. 5. how many grams of the excess reagent in question 4 are left over?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
4. al2o3 (s) + 6hcl (aq) → 2alcl3 (aq) + 3h20(1) find the mass of alcl3 that is produced when 10.0 g...
Questions

Chemistry, 17.01.2020 12:31

History, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31


English, 17.01.2020 12:31


Health, 17.01.2020 12:31



Mathematics, 17.01.2020 12:31

English, 17.01.2020 12:31

History, 17.01.2020 12:31

Mathematics, 17.01.2020 12:31



Mathematics, 17.01.2020 12:31

Social Studies, 17.01.2020 12:31

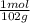 = 0,0980 moles
= 0,0980 moles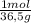 = 0,274 moles
= 0,274 moles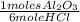 = 0,0457 moles of Al₂O₃
= 0,0457 moles of Al₂O₃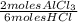 × 133
× 133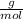 = 12,1 g of AlCl₃
= 12,1 g of AlCl₃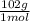 = 5,33 g of Al₂O₃
= 5,33 g of Al₂O₃ 


