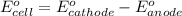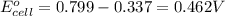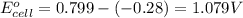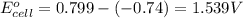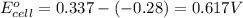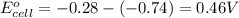Chemistry, 26.09.2019 16:30 savage5447
The standard reduction potentials of the following half-reactions are given in appendix e in the textbook:
ag+(aq)+e−→ag(s)= .799
cu2+(aq)+2e−→cu(s)= .337
ni2+(aq)+2e−→ni(s)= -.28
cr3+(aq)+3e−→cr(s). = -.74
1. determine which combination of these half-cell reactions leads to the cell reaction with the largest positive cell emf.
1st and 2nd,
1st and 3rd,
1st and 4th,
2nd and 3rd,
3rd and 4th.
it isn't the first or last one because i have gotten it wrong twice.
2. calculate the value of this emf.
3. then determine which combination is the smallest and calculate the emf.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
The standard reduction potentials of the following half-reactions are given in appendix e in the tex...
Questions



Engineering, 06.07.2019 03:20






Computers and Technology, 06.07.2019 03:20



Mathematics, 06.07.2019 03:20



History, 06.07.2019 03:20





Social Studies, 06.07.2019 03:20

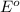 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.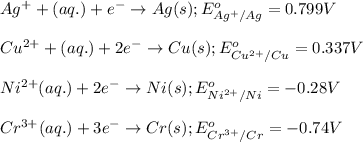
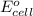 of the reaction, we use the equation:
of the reaction, we use the equation: