
Chemistry, 22.09.2019 03:20 chelseayazzie16
Asolution of heparin sodium, an anticoagulant for blood, contains 1.8 g of heparin sodium dissolved to make a final volume of 15 ml of solution. what is the mass/volume percent concentration of this solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3


You know the right answer?
Asolution of heparin sodium, an anticoagulant for blood, contains 1.8 g of heparin sodium dissolved...
Questions






English, 09.04.2021 01:00






Mathematics, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00

Chemistry, 09.04.2021 01:00


Mathematics, 09.04.2021 01:00


History, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00

History, 09.04.2021 01:00

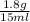 x 100 ml = 12 % m/v
x 100 ml = 12 % m/v

