
Chemistry, 22.09.2019 03:20 shealynh52
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (71.55 g/mol) and 20.0 g of magnesium metal? feof)+ mg() fe)mgo6) a) 62

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (7...
Questions

Mathematics, 20.03.2020 04:01






Mathematics, 20.03.2020 04:02

Mathematics, 20.03.2020 04:02

Health, 20.03.2020 04:02

Mathematics, 20.03.2020 04:02









English, 20.03.2020 04:03

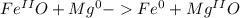
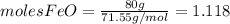 and
and 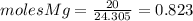 the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.
the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.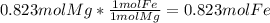 ). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe
). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe 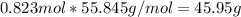 (molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron
(molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron


