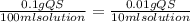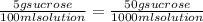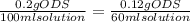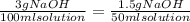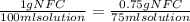Chemistry, 21.09.2019 01:30 LanaParrilla
12 instructions: • calculate both the volume and weight of each component to make the listed solutions. make sure to show your calculations for full credit. name both the volume or weight, units, and name of chemical for both the solute and the solvent. 1. 50ml of 1% acetic acid 2. 25ml of 10% sodium chloride 3. 10ml of 0.1% quinine sulfate 4. 1l of 5% sucrose 5. 60ml of 0.2% o-dianasidine in methanol 6. 50ml of 3% sodium hydroxide 7. 75ml of 1% sodium nitroferrocyanide

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
12 instructions: • calculate both the volume and weight of each component to make the listed soluti...
Questions



Biology, 22.09.2019 09:10

Social Studies, 22.09.2019 09:10

Mathematics, 22.09.2019 09:10


English, 22.09.2019 09:10


Chemistry, 22.09.2019 09:10

English, 22.09.2019 09:10



Mathematics, 22.09.2019 09:10

Social Studies, 22.09.2019 09:10

Health, 22.09.2019 09:10



Physics, 22.09.2019 09:10

History, 22.09.2019 09:10

Physics, 22.09.2019 09:10