
The elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 690 k is 1.27 × 101 m-1s-1. the reaction half-life at this temperature when [no2]0 = 0.45 m is s. the elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 690 k is 1.27 × 101 m-1s-1. the reaction half-life at this temperature when [no2]0 = 0.45 m is s. 0.17 18 5.7 0.054 0.079

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2

Chemistry, 23.06.2019 10:30
If a computer chip switches off -on-off in 0.015 us, what is the switching time in nanoseconds?
Answers: 2
You know the right answer?
The elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 6...
Questions

Biology, 10.12.2019 03:31

Social Studies, 10.12.2019 03:31

Mathematics, 10.12.2019 03:31


English, 10.12.2019 03:31

English, 10.12.2019 03:31


English, 10.12.2019 03:31

History, 10.12.2019 03:31

History, 10.12.2019 03:31


Mathematics, 10.12.2019 03:31


Mathematics, 10.12.2019 03:31

Mathematics, 10.12.2019 03:31

History, 10.12.2019 03:31

History, 10.12.2019 03:31

History, 10.12.2019 03:31


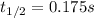
![\frac{1}{[NO_2]}=kt+ \frac{1}{[NO_2]_0}](/tpl/images/0248/0810/446e1.png)
![[NO_2]=\frac{[NO_2]_0}{2} \\](/tpl/images/0248/0810/70bce.png)
![t_{1/2}=\frac{1}{k[NO_2]_0} \\t_{1/2}=\frac{1}{(1.27x10^1\frac{L}{mol*s} )(0.45 \frac{mol}{L} )} \\\\t_{1/2}=0.175s](/tpl/images/0248/0810/f2ea3.png)


