

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

You know the right answer?
What is the freezing point of a 1,4-dioxane solution if that same solution boils at 104.6 °c? the n...
Questions

Mathematics, 15.07.2019 19:00





History, 15.07.2019 19:00



Mathematics, 15.07.2019 19:00



Mathematics, 15.07.2019 19:00

Mathematics, 15.07.2019 19:00

Chemistry, 15.07.2019 19:00






 °C
°C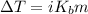
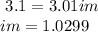
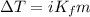
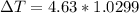
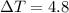 °C
°C

