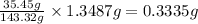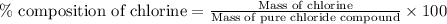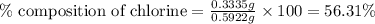Chemistry, 18.09.2019 03:30 shortcake8047
A0.5922 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride ion is precipitated as agcl by the addition of an excess of silver nitrate. the mass of the resulting agcl is found to be 1.3487 g. what is the mass percentage of chlorine in the original compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
A0.5922 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride...
Questions

Mathematics, 06.12.2020 23:30

Biology, 06.12.2020 23:30

History, 06.12.2020 23:30



Physics, 06.12.2020 23:30












Mathematics, 06.12.2020 23:30

Spanish, 06.12.2020 23:30