
Chemistry, 14.09.2019 10:10 mwms2018sg
You have a 0.5 l of a 1.0 m buffer solution with pka = 5.14 and current ph = 5.23. calculate the new ph when 1.50 ml of 5.00 m hcl is added. be mindful of units. (how can you tell if your answer makes sense? ) ph = pka + 109 u base 5.23 - 5.14+ log ( )

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2


You know the right answer?
You have a 0.5 l of a 1.0 m buffer solution with pka = 5.14 and current ph = 5.23. calculate the new...
Questions

Physics, 09.12.2019 18:31







Computers and Technology, 09.12.2019 18:31










Chemistry, 09.12.2019 18:31


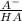
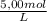 ÷ 0,5 L = 0,015 M of HCl
÷ 0,5 L = 0,015 M of HCl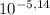
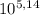 =
= ![\frac{[0,015M -x][0,55M-x]}{[0,45M-x]}](/tpl/images/0231/2479/0fe82.png)


