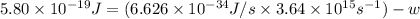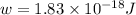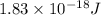Chemistry, 09.12.2019 18:31 gajdmaciej9502
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a kinetic energy of 5.80× 10–19 j. what is the maximum number of electrons that could be ejected from this metal by a burst of light (at some other frequency) with a total energy of 8.66× 10–7 j?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a ki...
Questions

English, 07.12.2020 16:40

Mathematics, 07.12.2020 16:40



English, 07.12.2020 16:40

Mathematics, 07.12.2020 16:40

Mathematics, 07.12.2020 16:40

Mathematics, 07.12.2020 16:40

Mathematics, 07.12.2020 16:40



Mathematics, 07.12.2020 16:40






Computers and Technology, 07.12.2020 16:40

English, 07.12.2020 16:40

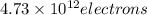
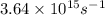
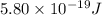
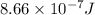
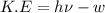
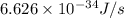
 = frequency
= frequency