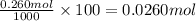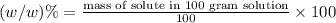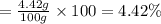Chemistry, 14.09.2019 09:10 toricepeda82
Use the ref an aqueous solution of chromium(ii) acetate has a concentration of 0.260 molal. the percent by mass of chromium(ii) acetate in the solution is submit answer retry entire group 9 more group attempts remaining

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
Use the ref an aqueous solution of chromium(ii) acetate has a concentration of 0.260 molal. the perc...
Questions

Mathematics, 21.09.2020 17:01



Mathematics, 21.09.2020 17:01

Mathematics, 21.09.2020 17:01

Mathematics, 21.09.2020 17:01




Medicine, 21.09.2020 17:01



Mathematics, 21.09.2020 17:01




Mathematics, 21.09.2020 17:01

Mathematics, 21.09.2020 17:01


Social Studies, 21.09.2020 17:01