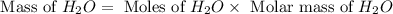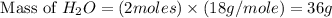Chemistry, 14.09.2019 08:30 anthonyhaywood
For the following reaction, 101 grams of magnesium nitride are allowed to react with 144 grams of water. mg3n2 (5) + 6 h20 (1) — 3 mg(oh)2 (aq) + 2 nh2 (aq) what is the formula for the limiting reagent? what is the maximum amount of magnesium hydroxide that can be formed? grams what amount of the excess reagent remains after the reaction is complete? grams submit answer retry entire group 8 more group attempts remaining

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
For the following reaction, 101 grams of magnesium nitride are allowed to react with 144 grams of wa...
Questions

English, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

Biology, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00



Mathematics, 25.05.2021 01:00











Mathematics, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

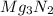 .
.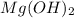 is, 174 grams.
is, 174 grams.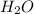 = 144 g
= 144 g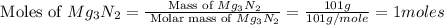
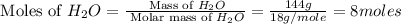
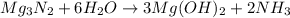
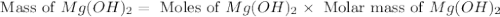
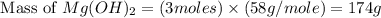
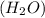 .
.