Chemistry, 14.09.2019 07:30 FailingstudentXD
Determine the theoretical yield of p2o5, when 3.07 g of p reacts with 6.09 g of oxygen in the following chemical equation
4 p + 5 o2 > 2 p2o5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Determine the theoretical yield of p2o5, when 3.07 g of p reacts with 6.09 g of oxygen in the follow...
Questions


Mathematics, 13.11.2020 07:00

Social Studies, 13.11.2020 07:00

Spanish, 13.11.2020 07:00

Computers and Technology, 13.11.2020 07:00

Chemistry, 13.11.2020 07:00



Mathematics, 13.11.2020 07:00

Mathematics, 13.11.2020 07:00



Mathematics, 13.11.2020 07:00

History, 13.11.2020 07:00

History, 13.11.2020 07:00

Biology, 13.11.2020 07:00




Health, 13.11.2020 07:00

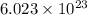 of particles.
of particles.
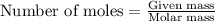
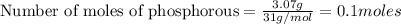
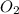 = 6.09 g
= 6.09 g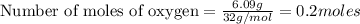
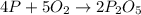
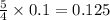 moles of oxygen
moles of oxygen 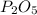
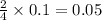 moles of
moles of