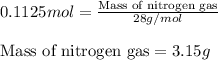Chemistry, 14.09.2019 07:30 qdogisbeast6132
For the following reaction, calculate how many grams of each product are formed when 4.05 g of water is used.
2 h20 > 2 h2 + o2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
For the following reaction, calculate how many grams of each product are formed when 4.05 g of water...
Questions

Mathematics, 21.04.2021 22:00


Engineering, 21.04.2021 22:00

Mathematics, 21.04.2021 22:00

Biology, 21.04.2021 22:00


Spanish, 21.04.2021 22:00



Business, 21.04.2021 22:00


Social Studies, 21.04.2021 22:00



French, 21.04.2021 22:00


Mathematics, 21.04.2021 22:00

Mathematics, 21.04.2021 22:00


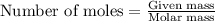 .....(1)
.....(1)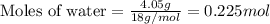
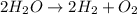
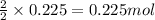 of hydrogen gas.
of hydrogen gas.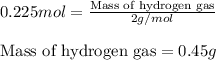
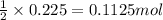 of nitrogen gas.
of nitrogen gas.