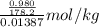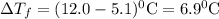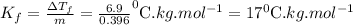A
pure solvent freezes at 12.0 c. a solution of 0.980 g of the solute
and 13.870 g of so...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
Questions

English, 30.05.2021 15:00

Mathematics, 30.05.2021 15:00

English, 30.05.2021 15:00

Law, 30.05.2021 15:00




Mathematics, 30.05.2021 15:00

Physics, 30.05.2021 15:00

History, 30.05.2021 15:00

Spanish, 30.05.2021 15:00

Spanish, 30.05.2021 15:00



Mathematics, 30.05.2021 15:00

Mathematics, 30.05.2021 15:00

Mathematics, 30.05.2021 15:00


Spanish, 30.05.2021 15:00

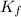 for solvent is
for solvent is 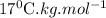
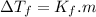 , where
, where 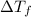 is depression in freezing point and m is molality of solution
is depression in freezing point and m is molality of solution