
Chemistry, 13.09.2019 22:30 sosick90501
Question: dimethylhydrazine, the
fuel used in the apollo lunar descentmodule, has a molar mass of
60.10 g/mol. it is made up of carbon, hydrogen, and nitrogen atoms.
the combustion of 2.859g of the fuelin excess oxygen yields 4.190g
of carbon dioxideand 3.428g ofwater. what are the simplest and
molecular formulas fordimethylhydrazine?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1



Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Question: dimethylhydrazine, the
fuel used in the apollo lunar descentmodule, has a molar mass...
fuel used in the apollo lunar descentmodule, has a molar mass...
Questions

History, 25.01.2022 01:20


Chemistry, 25.01.2022 01:20


Mathematics, 25.01.2022 01:20


Biology, 25.01.2022 01:20



Chemistry, 25.01.2022 01:20


English, 25.01.2022 01:20




Mathematics, 25.01.2022 01:20


Computers and Technology, 25.01.2022 01:20

Mathematics, 25.01.2022 01:20

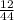 x 4.19g = 1.14g
x 4.19g = 1.14g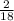 x 3.428g = 0.38g
x 3.428g = 0.38g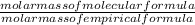 =
= 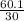 = 2
= 2

