

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Ahf [no2] = +33.2 kj mol. use the values below to calculate the standard molar enthalpy change for t...
Questions

Social Studies, 28.09.2019 11:30




History, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30


Computers and Technology, 28.09.2019 11:30

Geography, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30



Mathematics, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30



Mathematics, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30

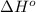
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0224/9686/45485.png)
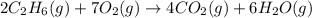
![\Delta H^o_{rxn}=[(4\times \Delta H^o_f_{(CO_2)})+(6\times \Delta H^o_f_{(H_2O)})]-[(2\times \Delta H^o_f_{(C_2H_6)})+(7\times \Delta H^o_f_{(O_2)})]](/tpl/images/0224/9686/92f41.png)
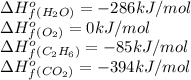
![\Delta H^o_{rxn}=[(4\times (-394))+(6\times (-286))]-[(2\times (-85))+(7\times 0)]\\\\\Delta H^o_{rxn}=-3122kJ](/tpl/images/0224/9686/cf6bf.png)


