
Chemistry, 06.09.2019 21:20 villafana36
The complete oxidation of one mole of a sugar produces carbon dioxide and water. 2000 kj of heat is transferred from the system to the surroundings. the rearrangement of bonds as 0.5 moles of the sugar are oxidized generates heat in an open test tube (101 j•l–1 pressure and 300 k temperature). what is the change in internal energy of the system (δu)? what is the change in enthalpy of the system(δh)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
The complete oxidation of one mole of a sugar produces carbon dioxide and water. 2000 kj of heat is...
Questions

Mathematics, 23.12.2020 19:40





Geography, 23.12.2020 19:40


English, 23.12.2020 19:50

Mathematics, 23.12.2020 19:50

Mathematics, 23.12.2020 19:50


English, 23.12.2020 19:50

Biology, 23.12.2020 19:50

Physics, 23.12.2020 19:50

Mathematics, 23.12.2020 19:50

History, 23.12.2020 19:50

Mathematics, 23.12.2020 19:50



Mathematics, 23.12.2020 19:50

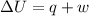
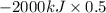
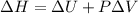
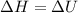
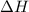 ) is -1000 kJ.
) is -1000 kJ.

