

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Consider the reaction of cacn2 and water to produce caco3 and nh3: cacn2 + 3 h2o → caco3 + 2 nh3 ....
Questions

Mathematics, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00



Mathematics, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00



Spanish, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

Biology, 02.03.2021 21:00

Chemistry, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

History, 02.03.2021 21:00


Mathematics, 02.03.2021 21:00

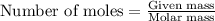

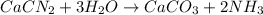
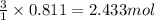 of water.
of water.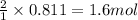 of ammonia.
of ammonia.

