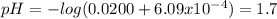A) a solution consists of 0.25 m hydrofluoric acid (hf) and 0.28 m sodium fluoride (naf). the k of hydrofluoric acid acid is 6.8 x 10"", calculate the ph of the solution. b) to one liter of the solution from part (a) is added 0.0200 moles of perchloric acid (hcio4). there is no change in the total volume. calculate the ph after the addition of the perchloric acid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation. 8. when a 2.5 mol of sugar (c12h22o11) are added to a certain amount of water the boiling point is raised by 1 celsius degree. if 2.5 mol of aluminum nitrate is added to the same amount of water, by how much will the boiling point be changed? show all calculations leading to your answer or use 3 – 4 sentences to explain your answer. 9. if 5.40 kcal of heat is added to 1.00 kg of water at 100⁰c, how much steam at 100⁰c is produced? show all calculations leading to an answer. 10. the freezing of water at 0⁰c can be represented as follows: h2o (l) ↔ h2o(s) the density of liquid water is 1.00 g/cm3. the density of ice is 0.92 g/cm3. in 3 – 4 sentences explain why applying pressure causes ice to melt.
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1


Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
A) a solution consists of 0.25 m hydrofluoric acid (hf) and 0.28 m sodium fluoride (naf). the k of h...
Questions

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

History, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

Mathematics, 13.09.2020 23:01

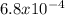 so you can write the equation:
so you can write the equation: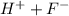
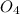 is a strong acid and completely dissociates.
is a strong acid and completely dissociates.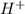 and x moles of
and x moles of 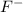 and you can write K like this:
and you can write K like this: ![K=\frac{[H^{+}][F^{-}] }{[HF]}](/tpl/images/0224/4748/809f2.png) . note that
. note that 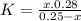 and solving for x, x=6.09x
and solving for x, x=6.09x 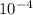 M
M![[H^{+} ]](/tpl/images/0224/4748/cd271.png) and you are abble to find pH with
and you are abble to find pH with ![pH=-log[H^{+}]=-log(6.09x10^{-4} )=3.2](/tpl/images/0224/4748/e6968.png)