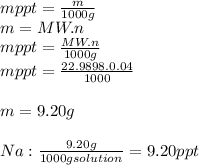
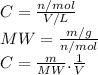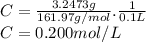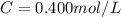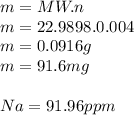3.2473 g of na2cro4 (mw = 161.97 g/mol) is dissolved in 100.0 ml of water. assuming the solution has a density of 1.00 g/ml, what is the concentration of na (mw = 22.9898 g/mol) in the solution in units of (a) molarity (m)? (b) parts per thousand (ppt)? (c) 10.0 ml of the solution is then diluted to a final volume of 1000.0 ml. what is the concentration of na in the diluted solution in units of parts per million (ppm)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2


You know the right answer?
3.2473 g of na2cro4 (mw = 161.97 g/mol) is dissolved in 100.0 ml of water. assuming the solution has...
Questions

Mathematics, 09.07.2021 19:50

Mathematics, 09.07.2021 19:50


Mathematics, 09.07.2021 19:50


Mathematics, 09.07.2021 19:50

History, 09.07.2021 19:50

Mathematics, 09.07.2021 19:50

Mathematics, 09.07.2021 19:50







Mathematics, 09.07.2021 19:50

Computers and Technology, 09.07.2021 19:50


Mathematics, 09.07.2021 19:50

Computers and Technology, 09.07.2021 19:50