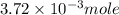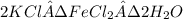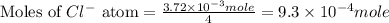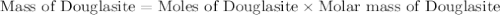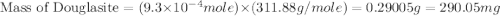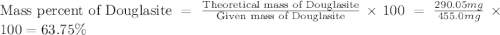Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2
You know the right answer?
Douglasite is a mineral with the formula 2kcl # fecl2 # 2h2o. calculate the mass percent of douglasi...
Questions



English, 03.01.2022 15:30

Geography, 03.01.2022 15:30


Mathematics, 03.01.2022 15:30




English, 03.01.2022 15:30

Geography, 03.01.2022 15:30

Health, 03.01.2022 15:30

Arts, 03.01.2022 15:30

English, 03.01.2022 15:30

History, 03.01.2022 15:40

Mathematics, 03.01.2022 15:40

English, 03.01.2022 15:40

Mathematics, 03.01.2022 15:40


English, 03.01.2022 15:40

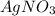 .
.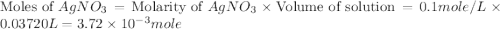
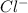 =
=