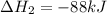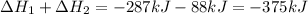Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

You know the right answer?
Calculate δh⁰298 (in kj) for the process p(s) + 5/2 cl2(g) → pcl5(g) from the following information....
Questions

Mathematics, 31.07.2019 00:50

History, 31.07.2019 00:50




Health, 31.07.2019 00:50

Mathematics, 31.07.2019 00:50






Physics, 31.07.2019 00:50




Mathematics, 31.07.2019 00:50


History, 31.07.2019 00:50

World Languages, 31.07.2019 00:50

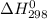 for the process is -375 kJ
for the process is -375 kJ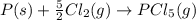 .
.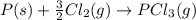 ......
......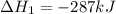
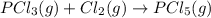 ......
......