
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as keq when a reaction is at equilibrium. (c) key does not change with temperature, whereas q is temperature dependent. (d) k does not depend on the concentration or partial pressures of reaction components. (e) q does not depend on the concentrations or partial pressures of reaction components.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1
You know the right answer?
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as...
Questions

Social Studies, 12.01.2020 12:31


Physics, 12.01.2020 12:31




Mathematics, 12.01.2020 12:31



Chemistry, 12.01.2020 12:31



Mathematics, 12.01.2020 12:31



History, 12.01.2020 12:31

Mathematics, 12.01.2020 12:31

History, 12.01.2020 12:31


Advanced Placement (AP), 12.01.2020 12:31

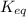 is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also,
is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also, 

