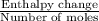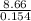In a coffee-cup calorimeter, 140.0 ml of 1.1 m and 140.0 ml of 1.1 m are mixed. both solutions were originally at 25.0°c. after the reaction, the final temperature is 32.4°c. assuming that all the solutions have a density of 1.0 and a specific heat capacity of 4.18 j/°c ⋅ g, calculate the enthalpy change for the neutralization of by . assume that no heat is lost to the surroundings or to the calorimeter. enthalpy change = kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
In a coffee-cup calorimeter, 140.0 ml of 1.1 m and 140.0 ml of 1.1 m are mixed. both solutions were...
Questions

Business, 17.08.2021 09:10

Mathematics, 17.08.2021 09:10

Mathematics, 17.08.2021 09:10



Mathematics, 17.08.2021 09:10

History, 17.08.2021 09:10

Mathematics, 17.08.2021 09:10

Biology, 17.08.2021 09:10

Physics, 17.08.2021 09:10

Mathematics, 17.08.2021 09:10


World Languages, 17.08.2021 09:10

Mathematics, 17.08.2021 09:10

English, 17.08.2021 09:10

Business, 17.08.2021 09:10

History, 17.08.2021 09:10


Mathematics, 17.08.2021 09:10

Business, 17.08.2021 09:10