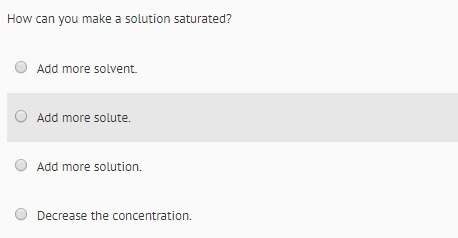
Chemistry, 03.09.2019 01:30 nessross1018
Dissolving 3.0 g of cacl2 in 150.0 g of water in a calorimeter (figure 5.12) at causes the temperature to rise to 25.8 c. what is the approximate amount of heat involved in the dissolution, assuming the heat capacity of the resulting solution is 4.18 j/gc? is the reaction exothermic or endothermic?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Dissolving 3.0 g of cacl2 in 150.0 g of water in a calorimeter (figure 5.12) at causes the temperatu...
Questions



Mathematics, 30.07.2021 01:00

Mathematics, 30.07.2021 01:00






Mathematics, 30.07.2021 01:00

Mathematics, 30.07.2021 01:00