
Chemistry, 03.09.2019 00:20 mahogany1956
Ammonia (nh3) reacts with oxygen to form nitric oxide (no) and water vapor: 4nh3 + 502 4no + 6h2o b) when 20.0 g nh3 and 50.0 g o2 are allowed to react, which is the limiting reagent? which reactant is left over and how much left over at the end of reaction? ans: 2.94 g c) what is the theoretical yield of no if 20.0 g nh3 and 50.0 g o2 are allowed to react? ans: 35.29 g d) what is the theoretical yield of h20 if 20.0 g nh3 and 50.0 g o2 are allowed to react? ans: 31.74 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3


Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

You know the right answer?
Ammonia (nh3) reacts with oxygen to form nitric oxide (no) and water vapor: 4nh3 + 502 4no + 6h2o b...
Questions


Mathematics, 26.10.2020 23:00

History, 26.10.2020 23:00

Mathematics, 26.10.2020 23:00

Mathematics, 26.10.2020 23:00

Biology, 26.10.2020 23:00

Social Studies, 26.10.2020 23:00


Health, 26.10.2020 23:00


Mathematics, 26.10.2020 23:00



Mathematics, 26.10.2020 23:00


English, 26.10.2020 23:00




Health, 26.10.2020 23:00

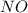 is 35.29 g.
is 35.29 g.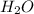 is 31.74 g.
is 31.74 g.
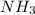
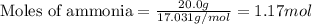
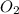
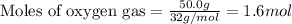
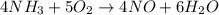
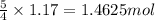 of oxygen
of oxygen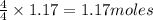 of
of 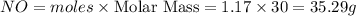
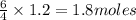 of
of 


