

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2


Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
Lead(ii) nitrate is added slowly to a solution that is 0.0800 m in ct ions. calculate the concentrat...
Questions

Biology, 19.09.2019 07:30

Spanish, 19.09.2019 07:30



Mathematics, 19.09.2019 07:30

History, 19.09.2019 07:30

Mathematics, 19.09.2019 07:30



Mathematics, 19.09.2019 07:30



English, 19.09.2019 07:30

Mathematics, 19.09.2019 07:30

Physics, 19.09.2019 07:30



Health, 19.09.2019 07:30


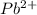 ion is 0.0375 M.
ion is 0.0375 M.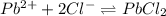
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0221/4126/7fd11.png)
![2.40\times 10^{-4}=[Pb^{2+}]\times (0.0800)^2](/tpl/images/0221/4126/e30e6.png)
![[Pb^{2+}]=0.0375M](/tpl/images/0221/4126/560e3.png)


